Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren


Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren
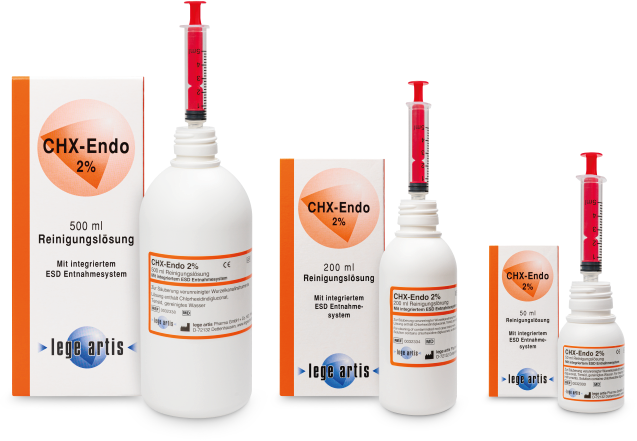
20% EDTA-Solution for locating, preparing and rinsing of root canals
Dosage form and pack size
ID: Medical Device (MD)
Downloads
Dosage form and pack size
ID: Medical Device (MD)
Downloads
Dosage form and pack size
ID: Medical Device (MD)
*Mohammadi Z; et al. Additive and reducing effects between calcium hydroxide and current irrigation solutions; The Journal of Contemporary Dental Practice VOL; 18(3); Page 246-249; 2017
Practical tip:
You can also mix CALCIPRO with saline solution, CHX solution or NaOCl solution!
Downloads
Expert Information
Safety Data Sheet
Summery of safety and clinical performance according to Article 32 MDR (in progress)
Downloads
Dosage form and pack size
ID: Medical Device (MD)
Downloads

Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren

Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren
Dosage form and pack size
ID: Medical Device (MD)
Downloads
Expert Information
Safety Data Sheet
Summery of safety and clinical performance according to Article 32 MDR (in progress)
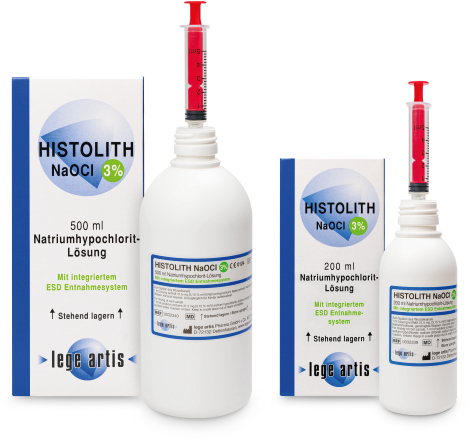
Dosage form and pack size
ID: Medicinal product (Registration number 6030426.00.00)
HISTOLITH NaOCl 5%. Dental Solution. Active ingredient: Sodium hypochlorite. 1 ml of solution contains 52.5 mg (5.25 % m/V) sodium hypochlorite corresponding to 50 mg (5.0 % m/V) active chlorine. Additives: Sodium chloride, sodium hydroxide and purified water. Indications: For cleaning and disinfection of root canals. Contraindications: Hypersensitivity to the active substance or any other ingredients or chlorine; open apical foramen. Side Effects: On vital tissue HISTOLITH NaOCl 5% effects highly caustic. In case of an inappropriate application (e.g. pressing the solution through the apex) this, because of its tissue-dissolving properties, may cause damages on vital tissue which comes in contact with NaOCl. Mostly, but not in every case, this damages are reversible. The following side effects have been reported (frequency not known): hypersensitivity, including swelling of face or mouth, oedema, inflammation, ulceration, necrosis, ecchymosis, pain, paresthesia and anesthesia affected facial nerves. Precautions: Caution, caustic. Date of current information: 10/2014
Practical tip:
Combined with CALCINASE EDTA-solution, you also remove the smear layer!
Downloads


Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren

Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren

Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren
Klicken Sie auf den unteren Button, um den Inhalt von zu laden.
Dosage form and pack size
ID: Medical Device (MD)
Practical tip:
Combined with CALCINASE EDTA-solution, you also remove the smear layer!
Downloads
Dosage form and pack size
ID: Medical Device (MD)
Practical tip:
Combined with CALCINASE EDTA-solution, you also remove the smear layer!
Downloads

Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren

Mit dem Laden des Videos akzeptieren Sie die Datenschutzerklärung von YouTube.
Mehr erfahren
Dosage form and pack size
ID: Medicinal product (Registration number 6031118.00.00)
TOXAVIT. Dental Paste. Active substances: paraformaldehyde, lidocaine hydrochloride 1 H2O and metacresol (Ph.Eur.). 1 g paste contains active ingredients: 460 mg paraformaldehyde, 370 mg lidocaine hydrochloride 1 H2O and 45 mg metacresol (Ph.Eur.). List of excipients: Eugenol, glycerol and carbon fibres. Therapeutic indications: TOXAVIT is used to devitalise dental pulp in particular cases where endodontic surgical measures (e.g. vital extirpation) are not possible. Prior to use, it should be checked whether successful treatment might be able to be achieved using other, aldehyde-free procedures (e.g. anaesthesia or bleeding control). Contraindications: Allergy to any of the active ingredients or any of the other ingredients. Undesirable effects: Uncommon: Upon extirpation of the pulp, occasional bleeding occurs at the apical site of detachment despite devitalisation. Very rare: When used on milk teeth, damage to the dental germ of the subsequent adult tooth may occur during the early stages of development (before mineralisation is complete) in extremely rare cases. Frequency not known: Following application to the exposed pulp cavity, more or less severe pulpitis-like complaints occur. These are relieved through the addition of the local anaesthetic lidocaine hydrochloride to the paste. In the event of insufficient diffusion or inadequate release of formaldehyde, vital tissue fragments can remain in the canal and cause considerable pain. If formaldehyde extravasates from the apex or the furcation area or side canals or leaking filling edges, inflammation or necrosis of periapical tissue, surrounding bone or the gums may result. Systemic effects cannot be ruled out. There are no findings on this method of application and local carcinogenicity. Local and systemic allergic reactions are possible. Date of current information: 12/2021. lege artis Pharma GmbH + Co. KG, P. O. Box 60, D-72132 Dettenhausen.
Downloads
| Ist CALCINASE EDTA-Lösung apothekenpflichtig? | Nein |
| Ist CALCINASE EDTA-Lösung verschreibungspflichtig? | Nein |
| IST CALCINASE EDTA-Lösung gesondert abrechenbar? | Nein |
| Ist CALCINASE EDTA-Lösung ein Medizinprodukt? | Ja |
| Hat CALCINASE EDTA-Lösung eine Pharmazentralnummer (PZN)? | Nein |
| Hat CALCINASE EDTA-Lösung einen EAN-13 Barcode (European article number)? | Ja, ist auf die Faltschachtel aufgedruckt (426009703 301 5 / 426009703 332 9 / 426009703 331 2 für 50/200/500 ml) |
| Gibt es für CALCINASE EDTA-Lösung ein Sicherheitsdatenblatt (SDB)? | Ja; in Deutsch und Englisch (als PDF-Datei unter „Downloads“ abrufbar) |
| Gibt es Veröffentlichungen zu CALCINASE EDTA-Lösung? | Ja; Sonderdrucke können beim Hersteller angefordert werden |
| Wie lange ist CALCINASE EDTA-Lösung haltbar? | 3 Jahre ab Herstellung (siehe Angaben auf Etikett und Faltschachtel) |
| Gibt es CALCINASE EDTA-Lösung in verschiedenen Größen? | Ja; CALCINASE EDTA-Lösung ist in der 50 ml, 200 ml und 500 ml Flasche erhältlich |
| Welche EDTA-Konzentration hat CALCINASE EDTA-Lösung? | CALCINASE EDTA-Lösung enthält 20 % Natriumedetat |
| Wie wirkt CALCINASE EDTA-Lösung? | EDTA löst Calcium-Ionen durch Ausbildung eines Chelat-Komplexes aus der Wurzelkanaloberfläche und erweicht damit den Untergrund bzw. entfernt den sogenannten smear-layer (Schmierschicht, die durch das Feilen entsteht); kann auch Kalk von Instrumenten lösen. Bei Sieben und empfindlichen Instrumenten erfolgt das Entkalken am besten durch Bürsten mit oder kurzes Einlegen in CALCINASE EDTA-Lösung. Anmerkung: Bitte die Pflegehinweise des Instrumentenherstellers beachten |
| CALCINASE EDTA-Lösung ist mir zu dünnflüssig, was kann ich tun? | Wem CALCINASE EDTA-Lösung zu dünnflüssig ist, kann die Verwendung eines EDTA-Gels, z.B. CALCINASE-slide (Art.-Nr. 0032319) empfohlen werden |
| Eignet sich CALCINASE EDTA-Lösung zur maschinellen Aufbereitung? | Möglicherweise besser geeignet hierfür ist z.B. CALCINASE-slide (Art.-Nr. 0032319), ein EDTA-Gel |
| Eignet sich CALCINASE EDTA-Lösung zur Wechselspülung? | Prinzipiell ja, z.B. mit HISTOLITH NaOCl 5% (50/200/500 ml; Art.-Nr. 0032111/0032120/0032112); HISTOLITH NaOCl 3% (200/500 ml; Art.-Nr. 0032339/0032340) oder HISTOLITH NaOCl 1% (200/500 ml; Art.-Nr. 0032342/0032343) andere Möglichkeiten müssen individuell geprüft werden) |
| Wann darf CALCINASE EDTA-Lösung nicht angewendet werden? | Bei Allergie gegen Natriumedetat; bei weit offenem Foramen apicale |
| Gibt es Nebenwirkungen bei CALCINASE EDTA-Lösung? | Bei bestimmungsgemäßem Gebrauch im Wurzelkanal keine bekannt. Sehr lange Einwirkzeiten oder langes Spülen mit großen Mengen an EDTA-Lösung können jedoch zur Erweichung des Wurzelden-tins und erhöhter Dentinpermeabilität führen. Gelangt Substanz über den Apex, sind Reizungen des periapikalen Gewebes möglich. |
| Kann ich CALCINASE-EDTA Lösung direkt in eine Spritze einfüllen? | Ja; durch das in alle Flaschen eingearbeitete ESD-System kann CALCINASE EDTA-Lösung direkt in eine Kunststoffspritze mit Luer- bzw. Luer Lock-System aufgezogen werden. |
| Kann CALCINASE EDTA-Lösung während Schwangerschaft und Stillzeit angewendet werden? | Ja, CALCINASE EDTA-Lösung ist hier aufgrund seiner Zusammensetzung unbedenklich anwendbar |
| Anwendung bei obliterierten (verkalkt) Wurzelkanal? | Erleichtert die mechanische Aufbereitung; kurzzeitige Einlage mit getränktem Wattefaden möglich |
| Wie hoch ist der pH-Wert von CALCINASE EDTA-Lösung? | 8,4 (Mittelwert; Spezifikation: 7,9 – 8,9 |
| Ist CALCINASE EDTA-Lösung apothekenpflichtig? | Nein |
| Ist CALCINASE EDTA-Lösung verschreibungspflichtig? | Nein |
| IST CALCINASE EDTA-Lösung gesondert abrechenbar? | Nein |
| Ist CALCINASE EDTA-Lösung ein Medizinprodukt? | Ja |
| Hat CALCINASE EDTA-Lösung eine Pharmazentralnummer (PZN)? | Nein |
| Hat CALCINASE EDTA-Lösung einen EAN-13 Barcode (European article number)? | Ja, ist auf die Faltschachtel aufgedruckt (426009703 301 5 / 426009703 332 9 / 426009703 331 2 für 50/200/500 ml) |
| Gibt es für CALCINASE EDTA-Lösung ein Sicherheitsdatenblatt (SDB)? | Ja; in Deutsch und Englisch (als PDF-Datei unter „Downloads“ abrufbar) |
| Gibt es Veröffentlichungen zu CALCINASE EDTA-Lösung? | Ja; Sonderdrucke können beim Hersteller angefordert werden |
| Wie lange ist CALCINASE EDTA-Lösung haltbar? | 3 Jahre ab Herstellung (siehe Angaben auf Etikett und Faltschachtel) |
| Gibt es CALCINASE EDTA-Lösung in verschiedenen Größen? | Ja; CALCINASE EDTA-Lösung ist in der 50 ml, 200 ml und 500 ml Flasche erhältlich |
| Welche EDTA-Konzentration hat CALCINASE EDTA-Lösung? | CALCINASE EDTA-Lösung enthält 20 % Natriumedetat |
| Wie wirkt CALCINASE EDTA-Lösung? | EDTA löst Calcium-Ionen durch Ausbildung eines Chelat-Komplexes aus der Wurzelkanaloberfläche und erweicht damit den Untergrund bzw. entfernt den sogenannten smear-layer (Schmierschicht, die durch das Feilen entsteht); kann auch Kalk von Instrumenten lösen. Bei Sieben und empfindlichen Instrumenten erfolgt das Entkalken am besten durch Bürsten mit oder kurzes Einlegen in CALCINASE EDTA-Lösung. Anmerkung: Bitte die Pflegehinweise des Instrumentenherstellers beachten |
| CALCINASE EDTA-Lösung ist mir zu dünnflüssig, was kann ich tun? | Wem CALCINASE EDTA-Lösung zu dünnflüssig ist, kann die Verwendung eines EDTA-Gels, z.B. CALCINASE-slide (Art.-Nr. 0032319) empfohlen werden |
| Eignet sich CALCINASE EDTA-Lösung zur maschinellen Aufbereitung? | Möglicherweise besser geeignet hierfür ist z.B. CALCINASE-slide (Art.-Nr. 0032319), ein EDTA-Gel |
| Eignet sich CALCINASE EDTA-Lösung zur Wechselspülung? | Prinzipiell ja, z.B. mit HISTOLITH NaOCl 5% (50/200/500 ml; Art.-Nr. 0032111/0032120/0032112); HISTOLITH NaOCl 3% (200/500 ml; Art.-Nr. 0032339/0032340) oder HISTOLITH NaOCl 1% (200/500 ml; Art.-Nr. 0032342/0032343) andere Möglichkeiten müssen individuell geprüft werden) |
| Wann darf CALCINASE EDTA-Lösung nicht angewendet werden? | Bei Allergie gegen Natriumedetat; bei weit offenem Foramen apicale |
| Gibt es Nebenwirkungen bei CALCINASE EDTA-Lösung? | Bei bestimmungsgemäßem Gebrauch im Wurzelkanal keine bekannt. Sehr lange Einwirkzeiten oder langes Spülen mit großen Mengen an EDTA-Lösung können jedoch zur Erweichung des Wurzelden-tins und erhöhter Dentinpermeabilität führen. Gelangt Substanz über den Apex, sind Reizungen des periapikalen Gewebes möglich. |
| Kann ich CALCINASE-EDTA Lösung direkt in eine Spritze einfüllen? | Ja; durch das in alle Flaschen eingearbeitete ESD-System kann CALCINASE EDTA-Lösung direkt in eine Kunststoffspritze mit Luer- bzw. Luer Lock-System aufgezogen werden. |
| Kann CALCINASE EDTA-Lösung während Schwangerschaft und Stillzeit angewendet werden? | Ja, CALCINASE EDTA-Lösung ist hier aufgrund seiner Zusammensetzung unbedenklich anwendbar |
| Anwendung bei obliterierten (verkalkt) Wurzelkanal? | Erleichtert die mechanische Aufbereitung; kurzzeitige Einlage mit getränktem Wattefaden möglich |
| Wie hoch ist der pH-Wert von CALCINASE EDTA-Lösung? | 8,4 (Mittelwert; Spezifikation: 7,9 – 8,9 |
| Ist CALCINASE-slide apothekenpflichtig? | Nein |
| Ist CALCINASE-slide verschreibungspflichtig? | Nein |
| IST CALCINASE-slide gesondert abrechenbar? | Nein |
| Ist CALCINASE-slide ein Medizinprodukt? | Ja |
| Hat CALCINASE-slide eine Pharmazentralnummer (PZN)? | Nein |
| Hat CALCINASE-slide einen EAN-13 Barcode (European article number)? | Nein |
| Gibt es für CALCINASE-slide ein Sicherheitsdatenblatt (SDB)? | Ja, in Deutsch und Englisch (als PDF-Datei unter „Download“ abrufbar) |
| Gibt es Veröffentlichungen zu CALCINASE-slide? | Ja; Sonderdrucke beim Hersteller anfordern |
| Wie lange ist CALCINASE-slide haltbar? | 3 Jahre ab Herstellung (siehe Angaben auf Etikett und Faltschachtel) |
| Gibt es CALCINASE-slide auch in anderen Größen? | Nein, nur als Packung mit 9 ml Tuben erhältlich |
| Welche EDTA-Konzentration hat CALCINASE-slide? | CALCINASE-slide enthält 15% Natriumedetat 15 % Natriumedetat in wasserlöslicher Gelgrundlage |
| Wie wirkt CALCINASE-slide? | EDTA löst Calcium-Ionen durch Ausbildung eines Chelat-Komplexes aus der Wurzelkanaloberfläche und erweicht damit den Untergrund bzw. entfernt den sogenannten smear-layer (eine Schmierschicht, die durch das Feilen entsteht); Gel dient als Gleitmittel für die verwendeten Feilen |
| Wie kann CALCINASE-slide entfernt werden? | Durch Spülen mit wässrigen Lösungen, z.B. HISTOLITH NaOCl 1%, 3% oder 5% oder physiolog. Kochsalzlösung |
| CALCINASE-slide ist mir zu dick, was kann ich tun? | Durch Scherung (z.B. durchspateln) kann das thixotrope Gel vorübergehend etwas dünn fließender gemacht werden; |
| Eignet sich CALCINASE-slide zur maschinellen Aufbereitung? | Ja; es wurde speziell für die maschinelle Aufbereitung entwickelt. |
| CALCINASE-slide haftet nicht auf Lentulo/Feile, was kann ich tun? | Gel kurz durchspateln, dann mit trockenem Lentulo bzw. Feile aufnehmen. |
| CALCINASE-slide ist eingedickt, hat sich verfestigt? | Wurde versehentlich die Kappe nicht fest aufgeschraubt oder die Tube längere Zeit offen gelassen, oder ist das Verfallsdatum überschritten, trocknet das Gel ein und wird unbrauchbar; versuchsweise kann es kurz vor Gebrauch durch Anmischen mit etwas Wasser oder besser CALCINASE- Lösung wieder in die gewünschte Konsistenz gebracht werden |
| Ist CALCINASE-slide röntgensichtbar? | Das Gel ist nicht röntgensichtbar |
| Gibt es Nebenwirkungen bei CALCINASE-slide? | Bei bestimmungsgemäßem Gebrauch im Wurzelkanal sind keine bekannt; gelangt Gel über den Apex, sind Reizungen des periapikalen Gewebes möglich |
| Wann darf CALCINASE-slide nicht angewendet werden? | Bei Allergie gegen Natriumedetat; bei weit offenem Foramen apicale. |
| Hilfsmittel zur Applikation von CALCINASE-slide? | Kann direkt aus der Tube entnommen werden. |
| Ist CALCIPRO apothekenpflichtig? | Nein |
| Ist CALCIPRO verschreibungspflichtig? | Nein |
| Ist CALCIPRO abrechenbar? | Nein |
| Ist CALCIPRO ein Medizinprodukt? | Ja |
| Hat CALCIPRO eine Pharmazentralnummer (PZN)? | Ja, PZN 03672148 |
| Hat CALCIPRO einen EAN-13 Barcode (European article number)? | Ja, ist auf Faltschachtel aufgedruckt (4 260097 033183) |
| Gibt es für CALCIPRO ein Sicherheitsdatenblatt (SDB)? | Ja, in Deutsch und Englisch (als PDF-Datei unter „Download“ abrufbar) |
| Gibt es Veröffentlichungen zu CALCIPRO? | Anwenderbericht |
| Wie lange ist CALCIPRO haltbar? | 3 Jahre ab Herstellung (s. Angaben auf Etikett und FS); Flasche gut verschlossen halten |
| Gibt es CALCIPRO auch als Paste? | Nein |
| Ist CALCIPRO röntgensichtbar? | CALCIPRO ist durch die enthaltenen Bestandteile röntgensichtbar; die Röntgensichtbarkeit liegt im Bereich von Dentin/Schmelz. Die Röntgensichtbarkeit von CALCIPRO ermöglicht die Qualitätskontrolle und erhöht die Anwendungssicherheit. |
| CALCIPRO härtet zu schnell aus? | Etwas mehr Flüssigkeit verwenden; etwas dünner anmischen. |
| CALCIPRO härtet zu langsam aus? | Etwas weniger Flüssigkeit verwenden; Verfallsdatum überschritten; zu lange offen gelassen. |
| Wie kann ich CALCIPRO wieder entfernen? | Hauptsächlich mechanisch durch Feilen und Reste chemisch durch Spülen mit z.B. CALCINASE EDTA-Lösung (Art.-Nr. 0032301, 0032331, 0032332) |
| Wann wende ich CALCIPRO an? | Zur direkten Überkappung der Pulpa sowie als temporäre Wurzelkanaleinlage. |
| Welches Mischungsverhältnis muss ich anwenden? Wie wird CALCIPRO angemischt? | So viel Pulver nach und nach zur Flüssigkeit zugeben, bis die gewünschte Konsistenz erreicht ist. |
| Mit was wird CALCIPRO angemischt? | Vorzugsweise mit dest. Wasser; in der Literatur wurde jedoch auch bereits von einer direkten Anmischung mit Natriumhypochloritlösung (z.B. HISTOLITH NaOCl 1%, 3% oder 5%) oder mit Chlorhexidinlösung zur besseren Wirkung gegen E. faecalis berichtet. |
| Welche Vorteile/Nachteile hat CALCIPRO gegenüber fertig angemischten Pasten? | Nachteile: kurzes Anmischen vor der Applikation nötig. Vorteile: frisch angemischtes Calciumhydroxid wirkt gleich bleibend gut (pH-Wert bleibt lange konstant), Verfestigt sich erst nach dem Anmischen und nicht bereits in der Spritze. |
| Ist CHX-Endo 2% Lösung apothekenpflichtig? | Nein |
| Ist CHX-Endo 2% Lösung verschreibungspflichtig? | Nein |
| Ist CHX-Endo 2% Lösung gesondert abrechenbar? | Nein |
| Ist CHX-Endo 2% Lösung ein Medizinprodukt? | Ja |
| Hat CHX-Endo 2% Lösung eine Pharmazentralnummer (PZN)? | Nein |
| Hat die CHX-Endo 2% Lösung einen EAN-13 Barcode (European article number)? | Ja, ist auf Faltschachtel aufgedruckt (200 ml 4 260097 033343 / 500 ml 4 260097 033381) |
| Gibt es für die CHX-Endo 2% Lösung ein Sicherheitsdatenblatt (SDB)? | Ja, in Deutsch und Englisch (als PDF-Datei unter „Download“ abrufbar) |
| Gibt es Literatur zur CHX-Endo 2% Lösung? | Nein |
| Wie lange ist die CHX-Endo 2% Lösung haltbar? | 2 Jahre ab Herstellung (siehe Angaben auf Etikett und Faltschachtel) |
| Gibt es CHX-Endo 2% in verschiedenen Größen? | Ja; CHX-Endo 2% Lösung ist in der 200 ml und 500 ml Flasche erhältlich |
| Welche CHX-Konzentration hat CHX-Endo 2%? | CHX-Endo 2% Lösung enthält 2 % Chlorhexidindiglukonat |
| Wie wirkt die CHX-Endo 2% Lösung? | CHX-Endo 2% Lösung reinigt durch die enthaltenen Tenside die Feile im Stand oder mit der Lösung benetzte Oberflächen. Das Tensid Chlorhexidin vermindert gleichzeitig die Keimzahl der Endobakterien. |
| Eignet sich die CHX-Endo 2% Lösung zur maschinellen Aufbereitung? | CHX-Endo 2% reinigt und entkeimt auch Feilen während der maschinellen Aufbereitung |
| Eignet sich die CHX-Endo 2% Lösung zur Wechselspülung? | CHX-Endo 2% wurde nicht für diesen Zweck getestet. CHX-haltige und NaOCl-haltige Lösungen sollten nicht vermischt werden, da es hier zu rot gefärbten Ausfällungen kommt. Ebenso kann es beim Vermischen von EDTA-haltigen und CHX-haltigen Lösungen/Gelen zu einem weißen Niederschlag bzw. zur Ausbildung einer zweiten Phase kommen. |
| Kann die CHX-Endo 2% Lösung mit CALCIPRO angemischt werden? | CHX-Endo 2% wurde nicht für diesen Zweck getestet. In der Literatur wurde die Anwendung von Pasten aus CHX-Lösungen und Calciumhydroxidpulvern beschrieben. |
| Wann darf die CHX-Endo 2% Lösung nicht angewendet werden? | Bei Allergie gegen Chlorhexidin oder einen der Inhaltsstoffe |
| Gibt es Nebenwirkungen bei CHX-Endo 2%? | Bei bestimmungsgemäßem Gebrauch sind keine bekannt |
| Kann ich CHX-Endo 2% auch direkt mit einer Spritze entnehmen? | Ja; durch das in alle Flaschen eingearbeitete ESD-System kann CHX-Endo 2% direkt in eine Kunststoffspritze mit Luer- bzw. LuerLock- System eingesaugt werden. |
| Ist das ESD-Entnahmesystem patentiert? | Ist zum Patent angemeldet |
| Kann die CHX-Endo 2% Lösung während Schwangerschaft und Stillzeit angewendet werden | Ja, CHX-Endo 2% Lösung ist hier aufgrund seiner Zusammensetzung unbedenklich anwendbar |
| Kann CHX-Endo 2% auch im Wurzelkanal angewendet werden? (z.B. als Spülung) | CHX Endo 2% enthält keine dort nichtverwendbaren Stoffe. Alle dentalen Lösungen mit der Indikation „Zur Desinfektion des Wurzelkanals“ sind in Europa jedoch als Arzneimittel einzustufen und müssen dafür zugelassen werden (Beispiel HISTOLITH NaOCl 5%). Für CHX-Endo 2% wurde die Zulassung für diese Indikation aus Kostengründen nicht beantragt. |
| Wie hoch ist der pH-Wert von –CHX-Endo 2%? | 5,0 (Mittelwert; Spezifikation: 4,5 – 6,5) |
| Ist HERMETIC apothekenpflichtig? | Nein |
| Ist HERMETIC verschreibungspflichtig? | Nein |
| Ist HERMETIC gesondert abrechenbar? | Nein |
| Ist HERMETIC ein Medizinprodukt? | Ja |
| Hat HERMETIC eine Pharmazentralnummer (PZN)? | Nein |
| Hat HERMETIC einen EAN-13 Barcode (European article number)? | Ja, ist auf Faltschachtel aufgedruckt (HERMETIC 14 g Pulver: 4 260097 033091 und HERMETIC Lösung 8 ml 4 260097 033084) |
| Gibt es für HERMETIC ein Sicherheitsdatenblatt (SDB)? | Ja, in Deutsch und Englisch (als PDF-Datei unter „Download“ abrufbar) |
| Gibt es Veröffentlichungen zu HERMETIC? | Ja, Sonderdrucke können beim Hersteller angefordert werden |
| Wie lange ist HERMETIC haltbar? | Pulver und Lösung jeweils 3 Jahre ab Herstellung (s. Angaben auf Etikett und Faltschachtel) |
| HERMETIC bindet zu schnell ab | Möglicherweise sollten Sie etwas weniger Pulver zumischen |
| HERMETIC bindet zu langsam ab | Möglicherweise sollten Sie etwas mehr Pulver zumischen |
| HERMETIC bindet nicht ab | Produkt muss vom Hersteller überprüft werden; bitte zurücksenden |
| Ist HERMETIC röntgensichtbar? | Ja, Röntgensichtbarkeit ≥ 3 mm Aluminium (ISO 6876:2012) |
| Was passiert mit überfülltem Material? | Mengen überfüllten Materials werden absorbiert. |
| Ist HERMETIC zur retrograden Wurzelfüllung zu empfehlen? | Heutzutage werden dafür z.B. Glasionimerzemente (GIZ), verstärkte ZOE Zemente oder MTA (Mineral Trioxid Aggregate) empfohlen. |
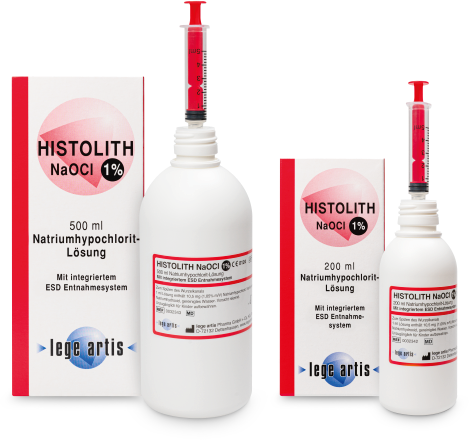
| Ist HISTOLITH NaOCl 1% apothekenpflichtig? | Nein |
| Ist HISTOLITH NaOCl 1% verschreibungspflichtig? | Nein |
| Ist HISTOLITH NaOCl 1% gesondert abrechenbar? | Nein |
| Ist HISTOLITH NaOCl 1% ein Medizinprodukt? | Ja |
| Hat HISTOLITH NaOCl 1% eine Pharmazentralnummer (PZN)? | Ja ist auf Faltschachtel aufgedruckt PZN 12472649 / PZN 12472655 (200 / 500 ml) |
| Hat HISTOLITH NaOCl 1% einen EAN-13 Barcode (European article number)? | Ja, 426009703 342 8 / 426009703 343 5 (200 / 500 ml) |
| Gibt es für HISTOLITH NaOCl 1% ein Sicherheitsdatenblatt (SDB)? | Ja, in Deutsch und Englisch (als PDF-Datei unter „Download“ abrufbar) |
| Gibt es Veröffentlichungen zu HISTOLITH NaOCl 1%? | Anwenderberichte noch keine vorhanden |
| Wie lange ist HISTOLITH NaOCl 1% haltbar? | HISTOLITH NaOCl 1% 200 ml ist 2 Jahre haltbar und HISTOLITH NaOCl 1% 500 ml ist 3 Jahre haltbar (ungeöffnet, Lagerung bei 2 – 25 °C). Die Verwendungsdauer nach Anbruch der Flasche beträgt 6 Monate. Lagerung stehend, nur in den Originalbehältern. |
| Wie wird die Haltbarkeit von HISTOLITH NaOCl 1% beeinflusst? | Das Behältnis ist stets gut verschlossen zu halten, da sonst mit einem Absinken der NaOCl-Konzentration zu rechnen ist. |
| Kann ich HISTOLITH NaOCl 1% erwärmen? | Wird in der Literatur zur Steigerung der Effektivität beschrieben. Falls erwärmt wird, sollte nur die benötigte Menge (z.B. eine zuvor gefüllte Spritze) möglichst kurz und rasch erwärmt werden und die Lösung unmittelbar danach angewendet werden, da durch die Aktivierung der Lösung der Gehalt dann schnell abnimmt. |
| Kann ich HISTOLITH NaOCl 1% mit Ultraschall anwenden? | Wird in der Literatur zur Steigerung der Effektivität beschrieben. Falls Ultraschall angewendet wird, sollte dies direkt im Kanal stattfinden, da durch die Aktivierung der Lösung der Gehalt dann schnell abnimmt. |
| Enthält HISTOLITH NaOCl 1% Stabilisatoren? | Ja, Natriumhydroxid und Natriumchlorid. |
| Kann ich HISTOLITH NaOCl 1% direkt in eine Spritze einfüllen? | Ja; durch das in alle Flaschen eingearbeitete ESD-System kann HISTOLITH NaOCl 1% direkt in eine Kunststoffspritze mit Luer- bzw. Luer Lock-System aufgezogen werden. |
| Ist Wechselspülung möglich? | Ja; z.B. Wechselspülung mit EDTA |
| Ist eine andere Konzentration von HISTOLITH NaOCl 1% erhältlich? | Ja; HISTOLITH NaOCl 3% und HISTOLITH NaOCl 5% |
| Was bedeutet die Bezeichnung NaOCl 1%? | Es ist ein Hinweis darauf, dass HISTOLITH NaOCl 1% Natriumhypochlorit (NaOCl) enthält. Die Prozentangabe bezieht sich auf die Menge aktiven Chlors in mg pro ml (m/V). |
| Kann ich HISTOLITH NaOCl 1% auch als Mundspüllösung verwenden? | Nein, HISTOLITH NaOCl 1% ist dafür nicht geeignet |
| Kann ich HISTOLITH NaOCl 1% zur Taschenspülung verwenden? | Für dieses Anwendungsgebiet ist HISTOLITH NaOCl 1% nicht klinisch geprüft worden und daher ist dies nicht Bestandteil der Zweckbestimmung. Es gibt Hinweise aus der Literatur, dass verdünnte NaOCl-Lösungen (<1%) auch bereits zur Taschenspülung verwendet wurden. |
| Ist HISTOLITH NaOCl 3% apothekenpflichtig? | Nein |
| Ist HISTOLITH NaOCl 3% verschreibungspflichtig? | Nein |
| Ist HISTOLITH NaOCl 3% gesondert abrechenbar? | Nein |
| Ist HISTOLITH NaOCl 3% ein Medizinprodukt? | Ja |
| Hat HISTOLITH NaOCl 3% eine Pharmazentralnummer (PZN)? | Ja ist auf Faltschachtel aufgedruckt PZN 10832687 / PZN 10832693 (200 / 500 ml) |
| Hat HISTOLITH NaOCl 3% einen EAN-13 Barcode (European article number)? | Ja, 4 260097 033398 / 4 260097 033404 (200 / 500 ml) |
| Gibt es für HISTOLITH NaOCl 3% ein Sicherheitsdatenblatt (SDB)? | Ja, in Deutsch und Englisch (als PDF-Datei unter „Download“ abrufbar) |
| Gibt es Veröffentlichungen zu HISTOLITH NaOCl 3%? | Anwenderbericht: Dr. Zacek, Spülkonzept für den Praxisalltag (als PDF-Datei unter „Download“ abrufbar) |
| Wie lange ist HISTOLITH NaOCl 3% haltbar? | HISTOLITH NaOCl 3% ist 3 Jahre haltbar (ungeöffnet, Lagerung bei 2 – 8 °C). Die Verwendungsdauer nach Anbruch der Flasche beträgt 6 Monate. Lagerung stehend, nur in den Originalbehältern |
| Ist für HISTOLITH NaOCl 3% eine Kühlkette erforderlich? | HISTOLITH NaOCl 3% ist nicht kühlkettenpflichtig. Für HISTOLITH NaOCl 3% ist auf Basis der vorliegenden Daten und Erfahrungen eine Unterbrechung der Kühlung (bei Lagerung/Transport) bei 25 °C von max. einem Monat und bei 40°C von max. 7 Tagen ohne Qualitätseinbußen möglich. Zum Gebrauch kann die Flasche in der Praxis mehrmals aus dem Kühlschrank entnommen werden. |
| Wie wird die Haltbarkeit von HISTOLITH NaOCl 3% beeinflusst? | Das Behältnis ist stets gut verschlossen zu halten, da sonst mit einem Absinken der NaOCl-Konzentration zu rechnen ist |
| Kann ich HISTOLITH NaOCl 3% erwärmen? | Wird in der Literatur zur Steigerung der Effektivität beschrieben. Falls erwärmt wird, sollte nur die benötigte Menge (z.B. eine zuvor gefüllte Spritze) möglichst kurz und rasch erwärmt werden und die Lösung unmittelbar danach angewendet werden, da durch die Aktivierung der Lösung der Gehalt dann schnell abnimmt. |
| Kann ich HISTOLITH NaOCl 3% mit Ultraschall anwenden? | Wird in der Literatur zur Steigerung der Effektivität beschrieben. Falls Ultraschall angewendet wird, sollte dies direkt im Kanal stattfinden, da durch die Aktivierung der Lösung der Gehalt dann schnell abnimmt. |
| Enthält HISTOLITH NaOCl 3% Stabilisatoren? | Ja, Natriumhydroxid und Natriumchlorid |
| Kann ich HISTOLITH NaOCl 3% direkt in eine Spritze einfüllen? | Ja; durch das in alle Flaschen eingearbeitete ESD-System kann HISTOLITH NaOCl 3% direkt in eine Kunststoffspritze mit Luer- bzw. Luer Lock-System eingesaugt werden. |
| Ist Wechselspülung möglich? | Ja; z.B. Wechselspülung mit EDTA |
| Ist eine andere Konzentration von HISTOLITH NaOCl 3% erhältlich? | Ja; HISTOLITH NaOCl 1% und HISTOLITH NaOCl 5% |
| Was bedeutet die Bezeichnung NaOCl 3%? | Es ist ein Hinweis darauf, dass HISTOLITH NaOCl 3% Natriumhypochlorit (NaOCl) enthält. Die Prozentangabe bezieht sich auf die Menge aktiven Chlors in mg pro ml (m/V). |
| Kann ich HISTOLITH NaOCl 3% auch als Mundspüllösung verwenden? | Nein, HISTOLITH NaOCl 3% ist dafür nicht geeignet. |
| Kann ich HISTOLITH NaOCl 3% zur Taschenspülung verwenden? | Für dieses Anwendungsgebiet ist HISTOLITH NaOCl 3% nicht klinisch geprüft worden und daher ist dies nicht der Bestandteil der Zweckbestimmung. Es gibt Hinweise aus der Literatur, dass verdünnte NaOCl-Lösungen (<1%) auch bereits zur Taschenspülung verwendet wurden. |
| Was muss ich tun wenn mir HISTOLITH NaOCl 3% ins Auge spritzte? | Rasch mit viel Wasser ausspülen. Optimal wäre die Spülung mit Hilfe einer Augenwaschflasche. Bei Bedarf (und zur zusätzlichen Sicherheit) anschließend den Arzt bzw. Augenarzt konsultieren. |
| Umrechnung aktives Chlor in Natriumhypochlorit? | 3 % m/V aktives Chlor entspricht 3,15 % m/V NaOCl |
| Umrechnung m/V in m/m? | Abhängig von der Dichte der Lösung. Diese liegt bei etwa 1.06 g/ml. Damit 3,15 % m/V NaOCl in etwa 3,0 % m/m (oder Gew.-%) NaOCl. Umgekehrt sind 3 Gew.-% in etwa 3,2 % m/V. |
| Kann ich HISTOLITH NaOCl 3% mit chlorhexidinhaltigen Produkten verwenden? | Chlorhexidinhaltige Produkte sollten nicht unmittelbar mit NaOCl-haltigen Produkten wie HISTOLITH NaOCl 3% kombiniert werden, da sonst ein unerwünschter schwerlöslicher roter Niederschlag entsteht. Hier muss stets eine ausreichende Zwischenspülung erfolgen. |
| Wie hoch ist der pH-Wert von HISTOLITH NaOCl 3%-Lösung? | ca. 12 (Spezifikation: 11,0 – 13,0) |
| Wann darf HISTOLITH NaOCl 3% nicht angewendet werden? | Bei Allergie gegen Chlor; bei offenem Foramen apicale |
| Gibt es Nebenwirkungen bei HISTOLITH NaOCl 3% | Bei bestimmungsgemäßen Gebrauch im Wurzelkanal sind keine Nebenwirkungen bekannt. Gelangt Natriumhypochlorit über den Apex, sind Reizungen des periapikalen Gewebes möglich. Auf gesundes Gewebe wirkt HISTOLITH NaOCl 3% reizend. |
| Wie wirkt HISTOLITH NaOCl 3% | Wird zur Erweiterung des Wurzelkanals vorher mit einer EDTA-Lösung gespült und anschließend HISTOLITH NaOCl 3% gereinigt, kann mit dieser Kombination die bei der Aufbereitung entstandene Schmier- und Belagschicht (smear layer) entfernt werden. |
| Ist HISTOLITH NaOCl 5% apothekenpflichtig? | Ja |
| Ist HISTOLITH NaOCl 5% verschreibungspflichtig? | Nein |
| Ist HISTOLITH NaOCl 5% gesondert abrechenbar? | Nein |
| Ist HISTOLITH NaOCl 5% ein Arzneimittel? | Ja |
| Hat HISTOLITH NaOCl 5% eine Pharmazentralnummer (PZN)? | Ja ist auf Faltschachtel aufgedruckt PZN 04869539 / PZN 05377850 ( 200 / 500 ml) |
| Hat HISTOLITH NaOCl 5% einen EAN-13 Barcode (European article number)? | Ja, 4 260097 031202 / 4 260097 031127 ( 200 / 500 ml) |
| Gibt es für HISTOLITH NaOCl 5% ein Sicherheitsdatenblatt (SDB)? | Ja, in Deutsch und Englisch (als PDF-Datei unter „Download“ abrufbar) |
| Gibt es Veröffentlichungen zu HISTOLITH NaOCl 5%? | Anwenderbericht: Dr. Lang; Die Konzentration muss stimmen (als PDF-Datei unter „Download“ abrufbar) |
| Wie lange ist HISTOLITH NaOCl 5% haltbar? | HISTOLITH NaOCl 5% ist im unversehrten Gefäß 3 Jahre ab Herstellung haltbar HISTOLITH NaOCl 5% ist nach Anbruch 6 Monate haltbar Lagerung stehend, nur in den Originalbehältern |
| Ist für HISTOLITH NaOCl 5% eine Kühlkette erforderlich? | HISTOLITH NaOCl 5% ist nicht kühlkettenpflichtig. Für HISTOLITH NaOCl 5% kann auf Basis der vorliegenden Daten und Erfahrungen eine Unterbrechung der Kühlung (bei Lagerung/Transport) von maximal einem Monat ohne Qualitätseinbußen angenommen werden. Kurzzeitige Entnahmen aus dem Kühlschrank zum jeweiligen Gebrauch sind mehrmals möglich. |
| Wie wird die Haltbarkeit von HISTOLITH NaOCl 5% beeinflusst? | Lagertemperatur: Höhere Temperaturen z.B. verringern die Haltbarkeit durch Abnahme der Wirkstoffkonzentration. Verunreinigungen: Möglichst nichts in die Flasche einbringen (Pipette, Spritzennadel etc., vor allem keine Metallgegenstände) sondern Umgießen! Einfacher und sicherer ist die direkte Befüllung von Kunststoffspritzen mit dem eingearbeiteten ESD-System. Damit kann die Haltbarkeit nach Öffnen verlängert werden Lagerungsart: Dunkel lagern, da Licht die Stabilität auch negativ beeinflusst. Da die Vorratsflaschen aber weiß opak sind, ist eine kurzzeitige Lagerung außerhalb des Kühlschranks ohne Einfluss auf die Stabilität. Aktivierung durch Wärme oder Ultraschall: danach nur noch kurzzeitig verwendbar, da die Konzentration durch die Aktivierung stark abnimmt. |
| Kann ich HISTOLITH NaOCl 5% erwärmen? | Wird in der Literatur zur Steigerung der Effektivität beschrieben. Falls erwärmt wird, sollte nur die benötigte Menge (z.B. eine zuvor gefüllte Spritze) möglichst kurz und rasch erwärmt werden und die Lösung unmittelbar danach angewendet werden, da durch die Aktivierung der Lösung der Gehalt dann schnell abnimmt. |
| Kann ich HISTOLITH NaOCl 5% mit Ultraschall anwenden? | Wird in der Literatur zur Steigerung der Effektivität beschrieben. Falls Ultraschall angewendet wird, sollte dies direkt im Kanal stattfinden, da durch die Aktivierung der Lösung der Gehalt dann schnell abnimmt. |
| Enthält HISTOLITH NaOCl 5% Stabilisatoren? | Ja, Natriumhydroxid und Natriumchlorid |
| Empfohlene Konzentration bei der Anwendung von HISTOLITH NaOCl 5%? | Zur Kanalaufbereitung unverdünnt (5 %) anwenden; in der Literatur sind jedoch auch geringere und höhere Konzentrationen beschrieben worden |
| Kann ich HISTOLITH NaOCl 5% direkt in eine Spritze einfüllen? | Ja; durch das in alle Vorratsflaschen eingearbeitete ESD-System kann HISTOLITH NaOCl 5% direkt in eine Kunststoffspritze mit Luer- bzw. Luer Lock-System aufgezogen werden. |
| Ist HISTOLITH NaOCl 5% verdünnbar? | Dies ist keine zugelassene Anwendung. NaOCl-Lösungen sind jedoch prinzipiell verdünnbar, z.B. mit destilliertem Wasser oder isoton. NaCl-Lsg.; jedoch rasch aufbrauchen. Die Wirkung verdünnter Lösungen wird in der Literatur kontrovers diskutiert. Es ist eher eine geringere Wirkung (Gewebs-auflösung; Desinfektion) zu erwarten. Es sollte dann länger gespült werden. |
| Ist HISTOLITH NaOCl 5% mischbar? | Nicht kompatibel mit den meisten anderen Flüssigkeiten. Bei sofortiger Anwendung eventuell möglich. Muss jedoch individuell ausprobiert werden. Neuerdings wird in der Literatur eine temp. Calciumhydroxid-Einlage, die zuvor mit NaOCl-Lsg. angemischt wurde, beschrieben. Könnte z.B. mit HISTOLITH NaOCl 5% und CALCIPRO probiert werden. |
| Ist Wechselspülung möglich? | Ja; z.B. Wechselspülung mit EDTA |
| Ist eine andere Konzentration von HISTOLITH NaOCl erhältlich? | Ja, HISTOLITH NaOCl 3% und HISTOLITH NaOCl 1% |
| Wirkung von HISTOLITH NaOCl 5%? | Desinfiziert und löst organisches Gewebe auf |
| Kann ich HISTOLITH NaOCl 5% auch als Mundspüllösung verwenden? | Nein, HISTOLITH NaOCl 5% ist dafür nicht geeignet |
| Kann ich HISTOLITH NaOCl 5% zur Taschenspülung verwenden? | Für dieses Anwendungsgebiet ist HISTOLITH NaOCl 5% nicht zugelassen. Es gibt Hinweise aus der Literatur, dass verdünnte NaOCl-Lösungen (<1%) auch bereits zur Taschenspülung verwendet wurden. |
| Was muss ich tun wenn mir HISTOLITH NaOCl 5% ins Auge spritzte? | Rasch mit viel Wasser ausspülen. Optimal wäre die Spülung mit Hilfe einer Augenwaschflasche. Bei Bedarf (und zur zusätzlichen Sicherheit) anschließend den Arzt bzw. Augenarzt konsultieren. |
| Was bedeutet die Bezeichnung NaOCl 5%? | Es ist ein Hinweis darauf, dass HISTOLITH NaOCl 5% Natriumhypochlorit (NaOCl) enthält. Die Prozentangabe bezieht sich auf die Menge aktiven Chlors in mg pro ml (m/V). |
| Umrechnung aktives Chlor in Natriumhypochlorit? | 5 % m/V aktives Chlor entspricht 5,25 % m/V NaOCl |
| Umrechnung m/V in m/m? | Abhängig von der Dichte der Lösung. Diese liegt bei etwas unter 1.1 g/ml. Damit 5,25 % m/V NaOCl in etwa 4,8 % m/m (oder Gew.-%) NaOCl. Umgekehrt sind 5 Gew.-% in etwa 5,5 % m/V. |
| Kann ich HISTOLITH NaOCl 5% mit Chlorhexidinhaltigen Produkten verwenden? | Chlorhexidinhaltige Produkte sollten nicht unmittelbar mit NaOCl-haltigen Produkten wie HISTOLITH NaOCl 5% kombiniert werden, da sonst ein unerwünschter schwerlöslicher roter Niederschlag entsteht. Hier muss stets eine ausreichende Zwischenspülung erfolgen. |
| Wie hoch ist der pH-Wert von HISTOLITH NaOCl 5%-Lösung? | ca. 12 (Spezifikation: 11,0 – 13,0) |
| Ist TOXAVIT apothekenpflichtig? | Ja |
| Ist TOXAVIT verschreibungspflichtig? | Nein |
| Ist TOXAVIT gesondert abrechenbar? | Ja; eventuell auch abrechenbar als Analgetikum |
| Ist TOXAVIT ein Arzneimittel? | Ja |
| Hat TOXAVIT eine Pharmazentralnummer (PZN)? | Nein |
| Hat TOXAVIT einen EAN-13 Barcode (European article number)? | Ja, ist auf Faltschachtel aufgedruckt (4 260097 031196) |
| Gibt es für TOXAVIT ein Sicherheitsdatenblatt (SDB)? | Ja, in Deutsch und Englisch (als PDF-Datei unter „Download“ abrufbar) |
| Gibt es Veröffentlichungen zu TOXAVIT? | Höke H. Paraformaldehydhaltige Devitalisierungsmittel. Dentalbarometer 2008; 2:26-27 Radl A. Devitalisierungsmittel – Quo vadis? ZMK 2008;24:161-163 |
| Wie lange ist TOXAVIT haltbar? | Im unversehrten Behältnis 2 Jahre ab Herstellung (siehe Angaben auf Etikett und Faltschachtel) nach Anbruch ist TOXAVIT innerhalb 6 Monaten aufzubrauchen |
| Ist TOXAVIT kühlkettenpflichtig? | Nein; für TOXAVIT kann auf Basis der vorliegenden Daten und Erfahrungen eine Unterbrechung der Kühlung (bei Lagerung/Transport) von maximal 3 Tagen ohne Qualitätseinbußen angenommen werden; kurzzeitige Entnahmen aus dem Kühlschrank zum jeweiligen Gebrauch sind mehrmals möglich. |
| Meine Kasse sagte mir, TOXAVIT sei nicht mehr zugelassen | TOXAVIT ist ein zugelassenes Arzneimittel; im Zuge der Nachzulassung wurde die Zulassung im September 2004 vom BfArM verlängert |
| Ich habe gehört/gelesen: TOXAVIT gibt es nicht mehr TOXAVIT wurde verboten TOXAVIT ist obsolet | TOXAVIT ist ein zugelassenes Arzneimittel und ist selbstverständlich weiterhin erhältlich. TOXAVIT ist weder verboten noch obsolet, seine Anwendung ist jedoch auf die Fälle beschränkt, in denen z.B. eine Vitalextirpation nicht möglich ist (siehe Angaben in der Gebrauchs- und Fachinformation unter „Anwendungsgebiete“). |
| TOXAVIT ist krümelig | Nach der Entnahme aus dem Kühlschrank sollte TOXAVIT erst auf Raumtemperatur kommen, um weicher zu werden und damit auch besser verarbeitet werden zu können. TOXAVIT kann nicht austrocknen. |
| Der Deckel ist lose oder undicht | Gelegentlich kann sich der Deckel des Originalitätsverschlusses nach Entfernen des Garantierings durch leichtes Entspannen des Kunststoffs etwas heben. Das Glas ist nach wie vor jedoch dicht, die Stabilität ist keinesfalls beeinträchtigt. |
| Wie viel TOXAVIT darf pro Anwendung verwendet werden? | Im Allgemeinen werden 20 – max. 25 mg TOXAVIT appliziert. Dies entspricht einem etwa Stecknadelkopf großen Kügelchen |
| Was muss ich bei der Anwendung von TOXAVIT beachten? | Während der Liegedauer ist ein dichter Verschluss der Kavität unbedingt erforderlich. Die Liegedauer von max. 14 Tagen vor Mortalextirpation soll nicht überschritten werden. Bei Vorliegen einer Perforation darf TOXAVIT nicht angewendet werden. Jeder Kontakt der paraformaldehydhaltigen TOXAVIT Paste mit dem umgebenden Weichgewebe beim Einbringen oder beim Verschluss des Zahnes durch Herausquellen ist wegen der stark ätzenden und nekrotisierenden Wirkung zu vermeiden |
| Wie lange darf die Liegedauer von TOXAVIT betragen? | Die Liegedauer von max. 14 Tagen vor Mortalextirpation soll nicht über- schritten werden. Bei Restvitalität kann die Anwendung unter Beachtung der Liegedauer von abermals max. 14 Tagen wiederholt werden. |
| Wann darf TOXAVIT nicht angewendet werden? | Bei Allergie gegen Formaldehyd, Lidocain, Metacresol und Eugenol. Bei Vorliegen einer Perforation darf TOXAVIT ebenfalls nicht angewendet werden |
| Ist TOXAVIT in der Schwangerschaft anwendbar? | Es liegen keine hinreichenden Daten für die Verwendung von TOXAVIT bei Schwangeren vor, daher ist bei einer Anwendung in der Schwangerschaft Vorsicht geboten. |
| Ist TOXAVIT in der Stillzeit anwendbar? | Es liegen keine hinreichenden Daten für die Verwendung von TOXAVIT bei stillenden Frauen vor, daher ist bei einer Anwendung Vorsicht geboten. |
| Fertilität, Schwangerschaft und Stillzeit | Aufgrund der anzunehmenden geringen systemischen Exposition von Paraformaldehyd nach topischer Anwendung von TOXAVIT sind keine Auswirkungen auf die Schwangerschaft oder das gestillte Neugeborene bzw. den gestillten Säugling zu erwarten. TOXAVIT kann in der Schwanger- schaft und bei stillenden Frauen mit der gebotenen Vorsicht angewendet werden. Für TOXAVIT liegen keine klinischen Studien über exponierte Schwangere vor. Tierexperimentelle Studien lassen nicht auf direkte oder indirekte schädliche Auswirkungen auf Schwangerschaft, embryonale/fetale Entwicklung, Geburt oder postnatale Entwicklung schließen (siehe Angaben in der Gebrauchs- und Fachinformation unter „Fertilität, Schwangerschaft und Stillzeit“). |
© Copyright 2021 lege artis Pharma GmbH + Co. KG | All rights reserved | Webdesign by Systeamhaus